New: Periodic Table points to tetrahedral nucleus
Moderators: Xen, expert, ChenBeier
New: Periodic Table points to tetrahedral nucleus
I need to discuss my idea:
Minor changes to Left Step Periodic Table lead to new discovery:
'spdf' blocks are nothing more than slices of the tetrahedron. Please check it out at www.perfectperiodictable.com. Click on 3D Image.
Stunning!
Minor changes to Left Step Periodic Table lead to new discovery:
'spdf' blocks are nothing more than slices of the tetrahedron. Please check it out at www.perfectperiodictable.com. Click on 3D Image.
Stunning!
Last edited by Valery on Thu Mar 06, 2008 3:57 pm, edited 1 time in total.
That seems like a good idea to search for
"Perfect Periodic Table ADOMAH PT" here:
http://www.webqc.org/chemicalsearch.php
I found a couple of sites right away.
"Perfect Periodic Table ADOMAH PT" here:
http://www.webqc.org/chemicalsearch.php
I found a couple of sites right away.
ADOMAH PT
You can find ADOMAH PT in 2D and 3D formats at www.perfectperiodictable.com. I highly recommend to read following pages: 'Description', '3D Image' and 'User guide'. Very interesting staff.
That is pretty amazing indeed!
Reading the website I would like to contest the following:
For atoms beyond hydrogen, quantum numbers n, l, ml & ms have only an approximate meaning. They are good for a qualitative description however.
[1] http://en.wikipedia.org/wiki/Quantum_number
[2] http://en.wikipedia.org/wiki/Hydrogen-like_atom
Reading the website I would like to contest the following:
Quantum numbers are result of an exact solution of the non-relativistic Schrödinger equation for a Hydrogen-like atom.quantum numbers n, l, ml and ms , that describe electronic populations of the atoms, are not completely understood in terms of mathematics
For atoms beyond hydrogen, quantum numbers n, l, ml & ms have only an approximate meaning. They are good for a qualitative description however.
[1] http://en.wikipedia.org/wiki/Quantum_number
[2] http://en.wikipedia.org/wiki/Hydrogen-like_atom
ADOMAH Tetrahedron
Thanks for your comment, Vitalii,
I always thought that the sets of the quantum numbers are the eigenvalues that the wave function depends on, not results of the solutions of the Schrodinger equation (which depend on the eigenvalues).
Also, I reviewed the web pages that you recommended. I do not think that the information presented there illustrates complete understanding of the quantum numbers and their interconnection. It rather illustrates lack of clarity. For example: aufbauprinzip requires the l = n-1, ..., 0 and not l = 0, ..., n-1. Why?
On the other hand, ADOMAH Tetrahedron Stack of spheres allows us to see the quantum numbers clearly as a system of coordinates that describe the tetrahedral structure. Moreover, there is no better explanation for the Madelung's (n+l) rule and aufbauprinzip than the tetrahedron packing, as demonstrated at www.perfectperiodictable.com on '3D Image' page.
Valery.
I always thought that the sets of the quantum numbers are the eigenvalues that the wave function depends on, not results of the solutions of the Schrodinger equation (which depend on the eigenvalues).
Also, I reviewed the web pages that you recommended. I do not think that the information presented there illustrates complete understanding of the quantum numbers and their interconnection. It rather illustrates lack of clarity. For example: aufbauprinzip requires the l = n-1, ..., 0 and not l = 0, ..., n-1. Why?
On the other hand, ADOMAH Tetrahedron Stack of spheres allows us to see the quantum numbers clearly as a system of coordinates that describe the tetrahedral structure. Moreover, there is no better explanation for the Madelung's (n+l) rule and aufbauprinzip than the tetrahedron packing, as demonstrated at www.perfectperiodictable.com on '3D Image' page.
Valery.
Best explanation for periods and length of periods is now on newly updated home page of www.perfectperiodictable.com.
It clearly explains why IUPAC Standard Periodic table does not "cut" sequence of the chemical elements correctly and violates quantum periodicity. The Periodic Law has never been explained so clearly!
Also, great quotation from Mendeleev's book.
It clearly explains why IUPAC Standard Periodic table does not "cut" sequence of the chemical elements correctly and violates quantum periodicity. The Periodic Law has never been explained so clearly!
Also, great quotation from Mendeleev's book.
Yes, that is definitely true.
I guess my point is that it is not the quantum numbers which are not well understood. It is the atomic properties can't be simply explained in terms of quantum numbers alone. One have to apply additional principles such as "Aufbau principle", second periodicity etc ...
Also I would like to comment on the following:
Vitalii
I guess my point is that it is not the quantum numbers which are not well understood. It is the atomic properties can't be simply explained in terms of quantum numbers alone. One have to apply additional principles such as "Aufbau principle", second periodicity etc ...
Also I would like to comment on the following:
The reason that those elements are not known is because their nuclei are not stable. Stability of nuclei is not related to the electronic configurations and therefore cannot be determined by periodicity rules found in periodic tables. In nuclear theory there might well be other periodic laws (and tetrahedron might also play an important role in explaining them) however they are not connected to the periodicity in chemical properties. In fact chemical properties are not affected by internal nuclear structure and only slightly affected by its mass (for a given nuclear charge).The reason for the absence of the elements with ground state electrons in subshells g, h, i and j corresponding to n = 5, 6, 7 and 8 is obvious: The Periodic Law follows the rule of the tetrahedron.
Vitalii
Yes, but.
Vitalii,
This is what I mean: for n=5 , l=4, 3, 2, 1, 0. There are no known elements that correspond to n=5 and l=4, that is, there are no elements with 5g electrons in ground state. Although element Z=93 exists with last electron in 5f, that is n=5, l=3. Same with 6f, 6g and 6h orbitals, as well as 7d, 7f, 7g, 7h and 7i. That is because Periodic Law does not follow "n", as quantum mechanics has it (2,8,18,32,50,72...), but follows "n+l" rule (2,2,8,8,18,18,32,32), which is not explained by the QM but explained by the tetrahedron sphere packing.
What you are saying further is absolutely correct from the modern science perspective:
This is the current state of chemistry where nucleus is regarded as a dot in space that has little to do with the electronic shells, the properties and the periodicity. But the fact that Periodic Law and the nucleus have connection to the tetrahedron, as demonstrated by the ADOMAH PT and ADOMAH Tetrahedron, Garai and Borg, points to a possibility of a more direct connection between the nucleus and the electronic shells than currently believed. This is exactly my point. It might be a long shot, but this could potentially revolutionize the way we view atoms !
This is what I mean: for n=5 , l=4, 3, 2, 1, 0. There are no known elements that correspond to n=5 and l=4, that is, there are no elements with 5g electrons in ground state. Although element Z=93 exists with last electron in 5f, that is n=5, l=3. Same with 6f, 6g and 6h orbitals, as well as 7d, 7f, 7g, 7h and 7i. That is because Periodic Law does not follow "n", as quantum mechanics has it (2,8,18,32,50,72...), but follows "n+l" rule (2,2,8,8,18,18,32,32), which is not explained by the QM but explained by the tetrahedron sphere packing.
What you are saying further is absolutely correct from the modern science perspective:
"In nuclear theory there might well be other periodic laws (and tetrahedron might also play an important role in explaining them) however they are not connected to the periodicity in chemical properties. In fact chemical properties are not affected by internal nuclear structure and only slightly affected by its mass (for a given nuclear charge)."!
This is the current state of chemistry where nucleus is regarded as a dot in space that has little to do with the electronic shells, the properties and the periodicity. But the fact that Periodic Law and the nucleus have connection to the tetrahedron, as demonstrated by the ADOMAH PT and ADOMAH Tetrahedron, Garai and Borg, points to a possibility of a more direct connection between the nucleus and the electronic shells than currently believed. This is exactly my point. It might be a long shot, but this could potentially revolutionize the way we view atoms !
New formulae
There are new formulae that demonstrate that all major parameters of a Periodic System can be determined if only one term is known:
edge of a regular tetrahedron that describes such Periodic System.
You can check it out at www.perfectperiodictable.com/novelty.
edge of a regular tetrahedron that describes such Periodic System.
You can check it out at www.perfectperiodictable.com/novelty.
3D Mnemonic diagram that shows the order of orbital filling
Typical mnemonic diagram that shows the order of orbital filling looks like this:
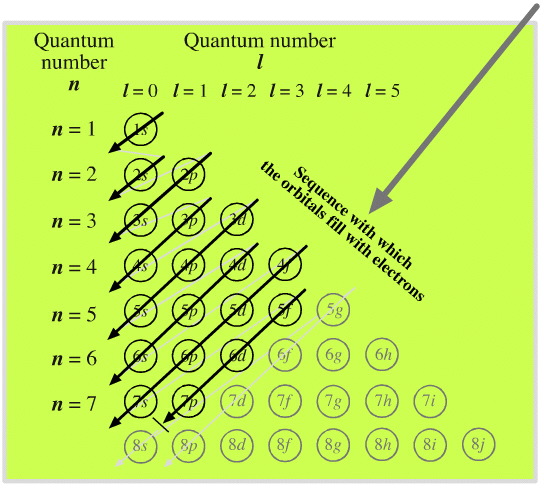
Such mnemonic diagrams have always been two dimensional: one dimension represents quantum number 'n' and the other dimension is quantum number "l".
New, three dimensional diagram, shows also magnetic quantum number "ml" in addition to "n" and "l". It demonstrates clearly how all three quantum nubers are related to each other. If interested, click HERE.

Such mnemonic diagrams have always been two dimensional: one dimension represents quantum number 'n' and the other dimension is quantum number "l".
New, three dimensional diagram, shows also magnetic quantum number "ml" in addition to "n" and "l". It demonstrates clearly how all three quantum nubers are related to each other. If interested, click HERE.


